You may or may not have read the last post, and if you’ve had high school Biology, there is no compulsion on you to read this one as well. In the last post, I talked fleetingly about the cell and its components: the cell membrane, mitochondria, nucleus, DNA, RNA, proteins, enzymes (which were also proteins), and the Central Dogma. This time, I want to talk about what makes a cell.
In this post, I would be covering carbohydrates, lipids (in the context of lipid bilayers), proteins, and nucleic acids (collective term for DNA and RNA).
Basically, I want to talk about Fried Chicken.
 One bite of this includes carbohydrates from the crisp, proteins from all the muscle inside, nucleic acids because you’re consuming the nuclei (singular: nucleus), and lipids (as fats) because well.. they’re fried.[1]
One bite of this includes carbohydrates from the crisp, proteins from all the muscle inside, nucleic acids because you’re consuming the nuclei (singular: nucleus), and lipids (as fats) because well.. they’re fried.[1]
(note: by the time I hit 1,500 words, I felt like this post should be chopped into two parts. Hence, the first part covers carbohydrates and lipids. The second would cover proteins, and the nucleic acids)
Carbohydrates
Carbo- comes from the word for coal in Latin, hydr- comes from the word for water (hudōr) in Greek, and -ate comes from the word for multiple oxygen atoms in Chemistry. So, carbohydrates are wet coal brimming with oxygens! Well.. almost.
Carbohydrates are called carbohydrates because they’re made up from carbon, hydrogen and oxygen atoms, and vary in many, many forms. In the simplest form, carbohydrates are found as monosaccharides (single sugars). You’ve probably heard of glucose-that’s one of them. Fructose, galactose and ribose are other examples of monosaccharaarsdsise monosaccharides (you don’t have to get the proper spelling on the first try, don’t worry). When two monosaccharides join together- we get a disaccharide (two sugars, you can probably see a pattern now). Cane sugar (or just.. sugar in English) is made from glucose and fructose linked together. Lactose (the thing you find in milk that makes Asians and old people sick)[2] is made from glucose and galactose linked together. Starch and fiber are polysaccharides and made of many such monosaccharides linked together.
(you don’t have to get the proper spelling on the first try, don’t worry). When two monosaccharides join together- we get a disaccharide (two sugars, you can probably see a pattern now). Cane sugar (or just.. sugar in English) is made from glucose and fructose linked together. Lactose (the thing you find in milk that makes Asians and old people sick)[2] is made from glucose and galactose linked together. Starch and fiber are polysaccharides and made of many such monosaccharides linked together.
Carbohydrates are the energy sources of life. You get yours from animals who get theirs from plants (or you can cut the middleman and grab it directly from plants). Plants, however, through science(!!!) and magic, MAKE their carbs from air, water, and SUNLIGHT (this is beyond cool). Carbohydrates are what make your food taste so good (hint: pizza, french fries, biryani, pad thai, muffins, cakes, donuts, chocolates, ice cream, and the list goes on endlessly), probably because you’ve evolved to like them. Carbs are broken down inside your stomach into simple sugars (monosaccharides), and then these simple sugars are further broken down in your mitochondria to release tremendous amounts of energy that you use to breathe, think, pump blood, digest, move, run, fight, talk, grow, repair, and basically do everything that makes you, you. This is achieved through a process called cellular respiration, which is beyond the scope of this blog (this is one of the things that I would not be excited to explain. Here’s a confession- I love Chemistry and Virology, NOT Biology).
Lipids
Lipids root from the Greek word for fat (lipos), however, lipids are NOT fats. Fats ARE lipids (bear with me, it’ll get clarified in a few).
Defining a lipid is much more harder than I anticipated. It turns out that there isn’t any well-defined concept of what a lipid is. Generally, they are small hydrophobic (this word means that they do NOT dissolve in water) molecules whose functions include energy storing, chemical signalling (means through which your cells talk to one another), and acting as structural components of the cell membrane (as lipid bilayers). They include fatty acids, fats, fat-soluble vitamins (A, D, E, K), waxes, phospholipids, steroids (like cholesterol) and much more. Fats are a subset of lipids- similar to how all trees are plants, yet not all plants are trees.
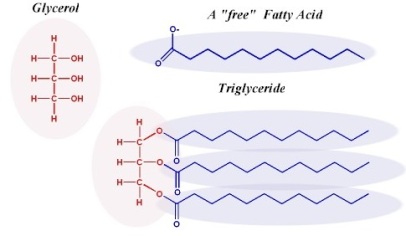
Fatty acids, even according to Wikipedia, are not to be confused with fats. They’re the building blocks of fats. A fatty acid is a carboxylic acid with a long (10+) aliphatic chain. Aliphatic compounds, opposed to aromatic compounds, are.. compounds that are not aromatic compounds. (Sorry for the circular loop, but I’d be more than willing to explain what they mean if you coerce me into doing so). They’re basically (I’m lying, but it’s fine for now) carbon atoms that are linked to other carbon atoms in a straight chain, without making a ring. The effect is shown below. The compound on the left is an aliphatic hexa-triene, and the one on the right is an aromatic cyclohexa-triene (also known as Mercedes Benzene).
When three fatty acids get linked to a glycerol molecule (shown on the left), you get a triglyceride. This is also a lipid, and a triglyceride in English is what we call ‘fat’. This is oil, butter, obesity, and whatever name you give to fat.
When two fatty acids and a phosphate group get linked to a glycerol molecule, you get a phospholipid. This is what forms the cell membrane (and the envelopes of viruses and organelles).
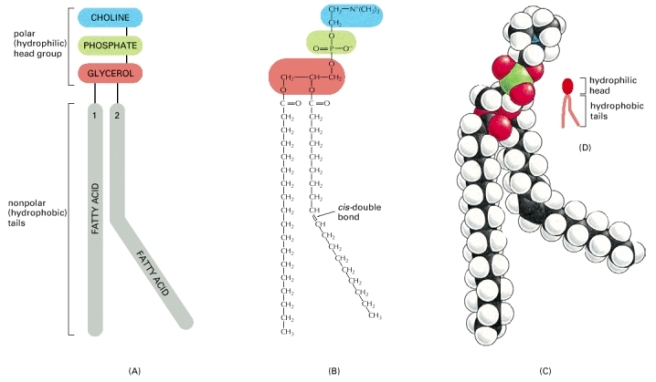
I get excited when Chemistry intersects with literally anything- so bear with me if I come off as too nerdy. Phospholipids are ‘amphiphilic’- meaning they have a hydrophobic and hydrophilic (water-loving) properties.
—- Start Of A Short Chemistry Lesson —-
If you’ve ever seen a cat scream while its in water- you can conclude the cat is hydrophobic. It doesn’t dissolve in water.
The properties of hydrophilicity and hydrophobicity arise because of an awesome property of Chemical bonds that we call polarity. To best explain the idea of polarity, I ask you to imagine a tug-of-war competition featuring a team of fifteen year-old students versus clones of Arnold Schwarzenegger. The rope would be lying unevenly, most of it towards team Arnold Schwarzenegger- and that situation is similar to how chemical bonds are made.
A chemical bond is made when atoms share electrons between themselves to fill valence shells (read: to complete each other- very, very romantic).[3] Some atoms, with a stronger force from the nucleus (the positive region), pull on those electrons (the rope) strongly compared to the other atom in the bond. The atoms who like pulling electrons (Team Arnold Schwarzenegger) are what we refer to as more electronegative atoms (because.. they like to be negatively charged). Then, there are atoms who like giving away those shared electrons (Team fifteen year-old), and we call them less electronegative. This creates a disparity across the chemical bond- and one side becomes slightly/partially (symbolized as δ-) negative, and the other side becomes slightly/partially (symbolized as δ+) positive.If the force is strong enough, the electrons in the bond can be completely stripped off from one atom, and we call that an ionic bond.

 This disparity in charge is called polarity, and it gives rise to hydrophobicity and hydrophilicity. In short, polar molecules tend to be hydrophilic. They love water so much that they’d rather dissolve in it (like table salt, alcohol, tea etc.). Less polar molecules (these arise when there isn’t a significant electronegativity difference between the atoms that form the bond, like a carbon-hydrogen bond) tend to be hydrophobic. They would rather dissolve in lipids instead of water (much like how cooking oil tends to float and form separate patches of oil on the surface of water- yet canola and olive oil mix together leaving no trace of such patches). Hydrophilicity and Hydrophobicity are very very important concepts in Biology, and they help explain key concepts like chemical reactivity, enzyme specificity (that’ll be covered later in Part 2), and even transport through the..
This disparity in charge is called polarity, and it gives rise to hydrophobicity and hydrophilicity. In short, polar molecules tend to be hydrophilic. They love water so much that they’d rather dissolve in it (like table salt, alcohol, tea etc.). Less polar molecules (these arise when there isn’t a significant electronegativity difference between the atoms that form the bond, like a carbon-hydrogen bond) tend to be hydrophobic. They would rather dissolve in lipids instead of water (much like how cooking oil tends to float and form separate patches of oil on the surface of water- yet canola and olive oil mix together leaving no trace of such patches). Hydrophilicity and Hydrophobicity are very very important concepts in Biology, and they help explain key concepts like chemical reactivity, enzyme specificity (that’ll be covered later in Part 2), and even transport through the..
PHOSPHOLIPID BILAYER!! (I can finally call the cell membrane as a phospholipid bilayer without people having to cringe at the name)
—- End Of A Short Chemistry Lesson —-
Lets return to the part where I said phospholipids are amphiphilic. The phospholipids have a hydrophilic head (of the phosphate group and glycerol), and a hydrophobic tail (of the two fatty acid chains). Now, you have to understand that opposites attract even in chemistry. The δ+ side prefers to face a δ- side if possible. However, hydrophilic (or polar) molecules prefer hydrophilic molecules and hydrophobic (or non-polar) molecules prefer hydrophobic molecules (in terms of what dissolves in what). This is NOT going against the opposites rule- so don’t be confused. This just means that when polar molecules are next to each other, they would reorient themselves such that the δ- side is paired against δ+ side and so on (dragging each other closer because they are then attracted). Similarly, if you put together a polar molecule against a non-polar molecule, the δ+/δ- side (of the polar molecule) has nothing on the other molecule (the non-polar molecule) to attract to. Through an oversimplification, this allows hydrophilic molecules to dissolve in water (a polar ‘solvent’) whereas hydrophobic molecules do not dissolve in water, and are soluble in fats and other non-polar solvents.
Imagine how you can make an infinitely long chain as long as you have enough hooks that are willing to go into loops- and loops that are willing to accommodate hooks (similar to how δ+ can link up with δ- infinitely). You can’t, however, fit the hook into anything that isn’t a loop (lets say, you can’t hook and attach a wall to the chain). Illustrated below, this phenomenon of polar molecules attracting each other (such as water molecules and acetone on the left) to form a compact shape is visible. In the case of 2-methyl propanone, it is non-polar, and it (although similar in size) occupies a larger space. This non-compatibility results in 2-methyl propanone being insoluble in water (or other polar solvents), and hence, hydrophobic.
 Anyways, moving away form the digressions, there is a certain property that arises because of the amphiphilicity and shape of the phospholipids, causes them to either form a or spherical micelle or a bilayer.[4] In each case, the polar side of the phospholipid is in contact with water, and the hydrophobic side is facing other hydrophobic sides of the same structure. Similarly, the nature of a bilayer restricts it to have any ‘free-edges’ that are exposed to water, and causes the bilayer to fold onto itself in a compartment. This very nature of the phospholipid bilayer gives the cell a shape. Otherwise, they’d probably look like sunny-side up eggs.
Anyways, moving away form the digressions, there is a certain property that arises because of the amphiphilicity and shape of the phospholipids, causes them to either form a or spherical micelle or a bilayer.[4] In each case, the polar side of the phospholipid is in contact with water, and the hydrophobic side is facing other hydrophobic sides of the same structure. Similarly, the nature of a bilayer restricts it to have any ‘free-edges’ that are exposed to water, and causes the bilayer to fold onto itself in a compartment. This very nature of the phospholipid bilayer gives the cell a shape. Otherwise, they’d probably look like sunny-side up eggs.

This formation of the cell membrane causes it to be semi-permeable. The lipid bilayer is permeable to both small and non-polar substances to pass. Ions (charged particles), and large polar molecules cannot pass through the membrane without assistance. This assistance is provided by cell-surface (or transmembrane) receptors on the cell, which regulate the transport of many biochemicals in and out of the cell.
 Here, I think we should stop. Initially I was planning to go through proteins and nucleic acids in the same post, but I think things were getting dense. So, all of that would be lumped in the second part of this post.
Here, I think we should stop. Initially I was planning to go through proteins and nucleic acids in the same post, but I think things were getting dense. So, all of that would be lumped in the second part of this post.
- Although Fried Chicken is extremely delicious, the author is not, in any way at all, encouraging you to consume Fried Chicken as a well-rounded diet.
- This was meant to be taken in light humor. I fully recognize that race has no basis in biology- however- statistics do show few trends that are retained genetically through races (or at least geographical distributions). Furthermore, this was meant to be taken as a stereotype since people of Jewish, African, and Native American descent have similar rates of lactose intolerant people as of East Asian descent. Anywho- since majority of the world’s adult population is lactose intolerant- it really can’t be lumped to any race. So yes, it makes East Asians sick, but it also makes everybody else sick (they’re just famous for it).
- I have made vast assumptions regarding background knowledge of valence shells, atoms, protons, electrons, and bonding. You can go here to understand covalent bonding. If you do not know what are atoms and sub-atomic particles (protons and electrons), feel free to contact me. I cannot make it simpler for the purpose of this blog, so I’d be more than willing to explain separately!
- This property is free energy minimization. Exposing the polar side to water allows energetically favorable bonds to form (releasing energy). Exposing the non-polar side to water causes water to form a cage-like structure (as shown in the picture) which is a more ‘orderly’ state for these water molecules (lowering entropy). Hence, free energy is minimized when the polar ends are exposed to water and the non-polar sides are away from water as much as possible. This is why the phosphate heads point away, and the fatty acid chains are facing each other.
For further reading:
Carbohydrates: Wikipedia, Simplified.
Cellular Respiration: Wikipedia, Simplified, SparkNotes, VERY Detailed.
Lipids: Wikipedia, Simplified, Detailed.
Covalent Bonding: Wikipedia, Sufficient (I love UCDavis), Detailed.
Polarity: Wikipedia, Sufficient.